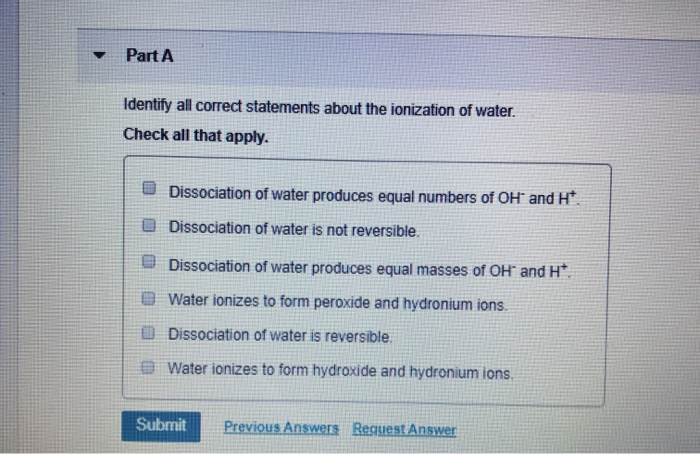
Identify All Correct Statements About the Ionization of Water
Neutral solutions contain no H3O ions. Check all that apply.

Ion Product Constant Chemistry Basics Science Chemistry Chemistry Notes
D Dissociation of water is reversible.
. Two water molecules interact to form a water molecule and a hydroxide ion. Identify all correct statements about the ionization of watercheck all that applycheck all that applywater ionizes to form peroxide and hydronium ionsdissociation of water produces equal numbers of oh- and hdissociation of water is reversiblewater ionizes to form hydroxide and hydronium ionsdissociation of water produces equal masses of oh- and. Identify all correct statements about the ionization of water.
Identify all correct statements about the ionization of water. Dissociation of water produces equal numbers of OH and H. Water ionizes to form hydroxide and hydronium ions.
HXaq MOHaq MXaq H 2O acid base salt ν If a strong base is neutralized with a strong acid the resulting solution contains only the salt HClaq NaOHaq NaCl. Neutral solutions contain no OH ions. Two water molecules interact to form two hydronium ions.
Identify all correct statements about the ionization of water-Dissociation of water is reversible-Dissociation of water produces equal numbers of OH- and H-Water ionizes to form peroxide and hydronium ions-Water ionizes to form hydroxide and hydronium ions-Dissociation of water produces equal masses of OH- and H. Dissociation of water produces equal numbers of OH- and H. C Dissociation of water produces equal numbers of OH- and H.
Click hereto get an answer to your question Which of the following statements isare correct about the ionic product of water. Dissociation of water produces equal masses of OH and H. Waters high surface tension is evident when certain insects are able to walk on the waters surface.
Which of the following statements are true. Which of the following statements is true for neutral solutions in which water is the solvent. Dissociation of water produces equal numbers of OH and H.
Identify all correct. Ionization energy and electron affinity both express the general attraction of an atom for electrons In general for representative elements atomic radii decreases from left to right across a period and increase down a group Match each type of ion with the correct description of its relative size-Cation. 2Dissociation of water produces equal numbers of OH- and H.
1Dissociation of water is reversible. Identify all correct statements about the ionization of water. Two water molecules interact to form a hydronium ion and a hydroxide ion.
E Water ionizes to form peroxide and hydronium. A Dissociation of water produces equal masses of OH- and H. Identify all correct statements about the ionization of water.
Water ionizes to form peroxide and hydronium ions. Check all that apply. This is true of any conjugate pair of acid and base Salts of Acids and Bases ν When an acid and a base undergo an exchange reaction the result is a salt and water.
Dissociation of water is not reversible. 3Water ionizes to form hydroxide and hydronium ions. Water ionizes to form peroxide and hydronium ions.
B Dissociation of water is not reversible. Neutral solutions contain H3O and OH ions in equal concentrations. Identify all correct statements about the ionization of water.
Dissociation of water produces equal masses of OH and H. Check all that apply. Water ionizes to form hydroxide and hydronium ions.
Dissociation of water is not reversible. Which of the following statements is. Which statement about the self-ionization of water is correct.
Waters surface tension results from the hydrogen bonds formed between the water molecules at the surface and between those surface molecules and the ones below.

Http Www Kangenwaterlifestyle Com Kangen Water Kangen Kangen Water Benefits

Solved Part A Identify All Correct Statements About The Chegg Com

Solved Identify All Correct Statements About The Ionization Chegg Com
No comments for "Identify All Correct Statements About the Ionization of Water"
Post a Comment